Take a look around — chances are, you’re surrounded by lithium-ion batteries. They’re in your phone, your laptop, your electric toothbrush, and maybe even your car. In today’s world, lithium-ion (Li-ion) batteries have silently become one of the most important technologies powering our everyday lives.
But what exactly is inside a lithium-ion cell? How does it work? And why is it so generally used?
In this article, we’re going to peel back the layers — literally — and dive deep into the structure, chemistry, and magic of lithium-ion batteries. Whether you’re a curious techie, an electrical hobbyist, or someone just wanting to understand how that power bank really works, you’re in the right place.
⚡ A Quick Intro: Why Lithium-Ion?
Before we get into the nuts and bolts, it’s important to understand why lithium-ion batteries are such a big deal.
Compared to older battery types like nickel-cadmium (NiCad) or lead-acid:
- They’re lighter
- They have a higher energy density
- They charge faster
- They last longer
- They don’t suffer from the feared “memory effect”
That’s why today’s smartphones don’t consider a ton and electric cars can actually replace gasoline.
🔋 What is a Lithium-Ion Cell?
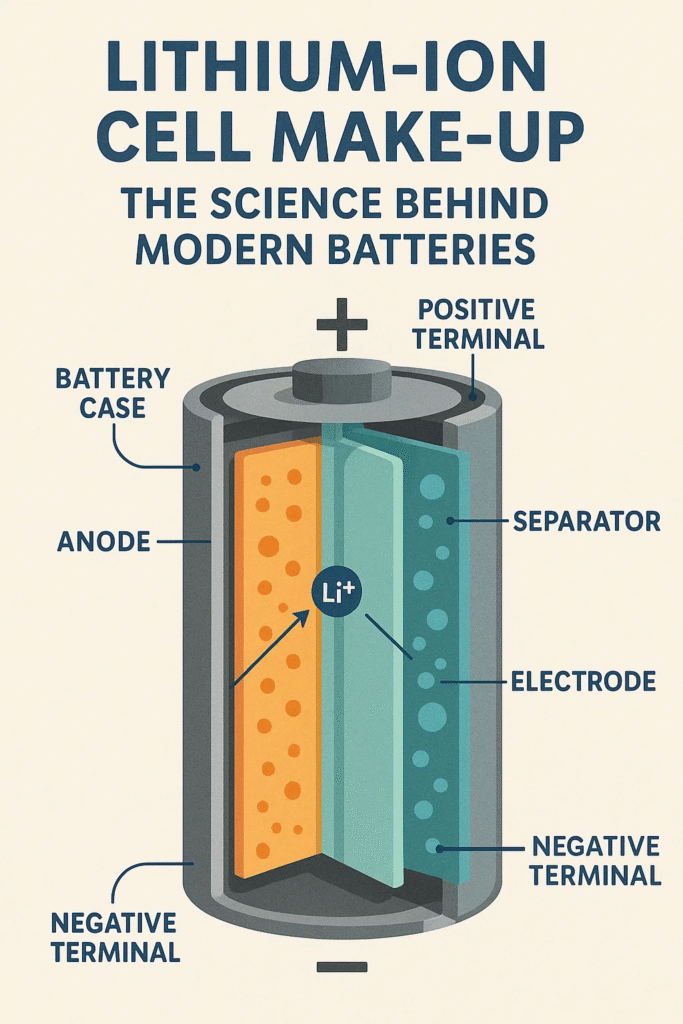
A lithium-ion cell is a rechargeable battery unit that works by moving lithium ions back and forth between two electrodes. Think of it like a two-way street — during charging, ions move in one direction; during discharging, they move in the other.
Each separate lithium-ion cell contains several parts:
- Cathode (positive electrode)
- Anode (negative electrode)
- Electrolyte
- Separator
- Current collectors
- Casing and protection components
Let’s break them all down.
🔴 The Cathode: Where the Power Starts
The cathode is the positive electrode of the battery. It’s made from a lithium metal oxide, such as:
- Lithium cobalt oxide (LiCoO₂)
- Lithium iron phosphate (LiFePO₄)
- Lithium nickel manganese cobalt oxide (LiNiMnCoO₂ or NMC)
Each of these has different properties depending on whether you’re designing a battery for a phone, an EV, or a solar backup system.
Why it matters:
The cathode is where lithium ions are stored when the battery is cleared. When you charge your battery, lithium ions move from here to the anode.
Pro tip: The choice of cathode material affects everything — from cost to safety to how fast it charges.
⚫ The Anode: Home for Lithium Ions
The anode is the negative electrode, and it’s commonly made from graphite, a form of carbon.
When you charge the battery, lithium ions travel from the cathode and embed themselves into the graphite layers of the anode — a process known as “intercalation.”
During discharge:
- Lithium ions move back to the cathode
- Electrons travel through the external circuit (powering your device)
Why it matters:
The anode plays a dangerous role in how much energy the battery can store and how fast it can relief it.
🧪 The Electrolyte: Ion Superhighway
The electrolyte is the medium that allows lithium ions to move between the cathode and anode. It usually consists of a lithium salt (like LiPF₆) dissolved in an organic solvent (like ethylene carbonate or dimethyl carbonate).
Why it matters:
This liquid (or sometimes gel or solid) must:
- Be a good conductor for ions
- Not conduct electrons
- Withstand high voltage
- Be chemically stable
Key point: Electrolytes are often flammable, which is why safety measures in battery design are so important.
🧻 The Separator: Safety First
Imagine a battery without a barricade between the anode and cathode — you’d basically have a short circuit and possibly a fire.
That’s where the separator comes in.
It’s a thin, porous plastic film (usually polyethylene or polypropylene) that keeps the electrodes apart while still allowing lithium ions to pass through.
Why it matters:
- Prevents internal short circuits
- Must be chemically stable in electrolyte
- Needs to shut down automatically if overheating (some separators melt to block ion flow)
🧲 Current Collectors: Guiding the Flow
On both sides of the battery, you’ll find current collectors:
- Aluminum foil for the cathode
- Copper foil for the anode
These materials collect electrons from the electrodes and channel them into the external circuit — aka, your device or car motor.
📦 Casing and Protection Circuitry
All these internal parts are enclosed in a metal or plastic casing — depending on the cell type:
- Cylindrical (like 18650 cells)
- Pouch (flat, flexible packs)
- Prismatic (rectangular blocks, common in EVs)
Plus, lithium-ion batteries often come with protection circuitry:
- To prevent overcharging
- To stop deep discharging
- To shut off if overheated or shorted
This is especially important in phones and laptops, where battery disappointment can be disastrous.
🔄 Charging & Discharging: What’s Really Happening?
Now let’s bring it all together.
🟢 Charging:
- External power is applied
- Lithium ions move from the cathode to the anode
- They embed themselves in the anode’s graphite layers
- Electrons flow through the external charger
🔴 Discharging:
- Lithium ions move back from anode to cathode
- Electrons flow through your device, providing power
This back-and-forth process can be repeated hundreds or even thousands of times, depending on battery quality.
📊 Energy Density, Cycle Life, and Efficiency
Here’s where things get interesting.
🔋 Energy Density:
This means how much energy a battery can store in a given size or weight.
- Li-ion batteries are great here — they pack more energy per gram than older chemistries.
🔁 Cycle Life:
This mentions to how many full charge-discharge cycles a battery can handle before its capacity drops significantly.
- Good lithium-ion cells can last 500–1500+ cycles.
⚙️ Efficiency:
Li-ion batteries typically have a charging efficiency of 95% or more. That means very little energy is lost in the process.
⚠️ Thermal Runaway: The Risk You Should Know
Even though lithium-ion batteries are generally safe, they’re not perfect.
The biggest danger is something called thermal runaway. This is when a battery overheats, and instead of cooling down, it gets hotter and hotter — leading to:
- Fire
- Explosion
- Toxic gas release
Common causes:
- Physical damage
- Industrial defects
- Overcharging
- High temperatures
That’s why battery management systems (BMS) are so important — they monitor temperature, voltage, and current to keep things safe.
🚗 Li-Ion Batteries in Electric Vehicles (EVs)
EVs like Tesla, Hyundai, or electric bikes all run on lithium-ion technology — but at a much larger scale.
- A Tesla Model S may have thousands of individual lithium-ion cells
- These are arranged into modules and packs
- Liquid or air cooling systems regulate temperature
- Stylish BMS ensures performance and safety
Why lithium-ion?
- Great range
- Fast charging
- Long life
- Lower cost over time compared to gas
But: Recycling and sourcing raw materials (like cobalt) are environmental challenges that need to be solved as EVs go mainstream.
🌱 Future of Lithium-Ion: Solid-State, Graphene, and Beyond
While lithium-ion is king today, researchers are working on even better options:
🔬 Solid-State Batteries:
- Replace liquid electrolyte with a solid one
- Safer, more stable, potentially higher energy density
- Still in early stages, but promising for future EVs
🔬 Graphene Anodes:
- Might increase charging speed
- Improve conductivity and lifespan
🔬 Silicon-based Anodes:
- Store up to 10x more lithium
- Might lead to major improvements in phone and car battery life
We’re at the edge of the next battery revolution — and lithium-ion is the bridge getting us there.
🤔 Frequently Asked Questions
Q1: Can you overcharge a lithium-ion battery?
Not if it’s protected. Most modern devices have BMS to prevent overcharging. But in raw cells, yes — and it’s dangerous.
Q2: Why do phone batteries degrade over time?
Every charge-discharge cycle causes micro damage inside the battery. Over time, capacity drops and resistance increases.
Q3: Should I let my battery drop to 0%?
No. It’s better to keep lithium-ion batteries between 20–80% for longer life.
Q4: What’s the difference between 18650 and 21700 batteries?
They’re both cylindrical lithium-ion cells — 21700 is newer, larger, and usually packs more energy.
📌 Conclusion
Lithium-ion batteries may seem like small, simple things, but under the surface, they’re full of brilliant engineering and captivating science. They’ve distorted how we live — from the phone in your hand to the car in your garage.
